Nicolas Gambardella · @nicgambarde
102 followers · 127 posts · Server genomic.socialRT @aSciStance@twitter.com
I have translated clinical trial and regulatory documents for so many of those medicines! Good to see that they are discussed by #CHMP
#FeelingUseful https://twitter.com/EMA_News/status/1617483638635114496
🐦🔗: https://twitter.com/aSciStance/status/1617500844047450113
Fabrizio Chiodo · @FabrizioChiodo
491 followers · 231 posts · Server mastodon.unoRT @EMA_News@twitter.com
The #CHMP recommended for authorisation the 1⃣st gene therapy to treat #haemophilia B in adults ⬇️
https://www.ema.europa.eu/en/news/first-gene-therapy-treat-haemophilia-b
Sophie111178 · @Sophie111178
104 followers · 107 posts · Server sueden.socialRT @U12Schutz
Die Tagesordnung für das #chmp Meeting der @EMA_News ist veröffentlicht. Neben neuen Medikamenten geht es auch wieder um Impfstoffe. https://twitter.com/EMA_News/status/1589573047736225792
Katja Adolf · @Katja_Adolf
62 followers · 52 posts · Server mstdn.scienceRT @BioNTech_Group@twitter.com
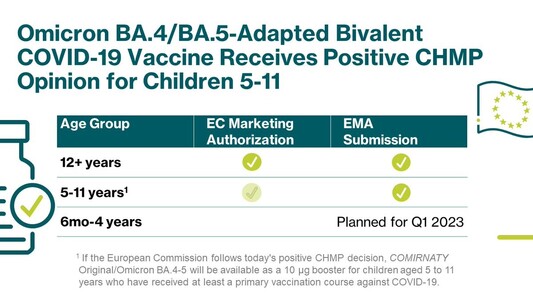
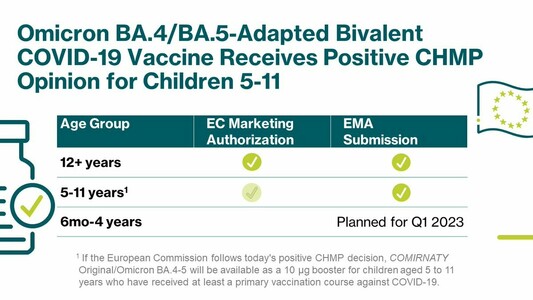
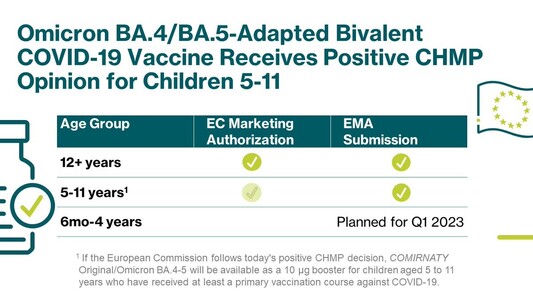
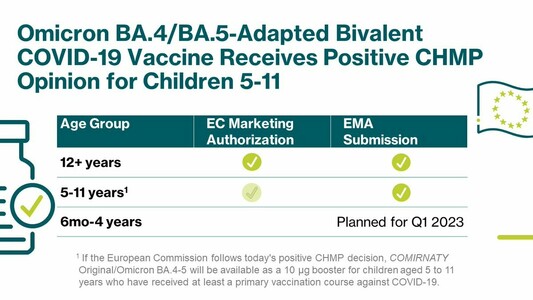
A booster dose of our Omicron BA.4/BA.5-adapted bivalent #COVID19 vaccine developed with @Pfizer@twitter.com has been recommended for marketing authorization by the @EMA_News@twitter.com’ #CHMP for children 5-11. https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-receive-positive-chmp-opinion-omicron-0
🐦🔗: https://twitter.com/BioNTech_Group/status/1590718161183047686
arminphilipp · @arminphilipp
684 followers · 218 posts · Server sueden.socialSchön, dass es dieses Mal schneller geht, auch für die Kids! 👍🏻
RT @BioNTech_Group@twitter.com
A booster dose of our Omicron BA.4/BA.5-adapted bivalent #COVID19 vaccine developed with @Pfizer@twitter.com has been recommended for marketing authorization by the @EMA_News@twitter.com’ #CHMP for children 5-11. https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-receive-positive-chmp-opinion-omicron-0
🐦🔗: https://twitter.com/BioNTech_Group/status/1590718161183047686
houseofpain · @houseofpain
33 followers · 414 posts · Server nrw.socialRT @BioNTech_Group@twitter.com
A booster dose of our Omicron BA.4/BA.5-adapted bivalent #COVID19 vaccine developed with @Pfizer@twitter.com has been recommended for marketing authorization by the @EMA_News@twitter.com’ #CHMP for children 5-11. https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-receive-positive-chmp-opinion-omicron-0
🐦🔗: https://twitter.com/BioNTech_Group/status/1590718161183047686
Sophie111178 · @Sophie111178
104 followers · 107 posts · Server sueden.socialRT @BioNTech_Group
A booster dose of our Omicron BA.4/BA.5-adapted bivalent #COVID19 vaccine developed with @pfizer has been recommended for marketing authorization by the @EMA_News’ #CHMP for children 5-11. https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-receive-positive-chmp-opinion-omicron-0
U12schutz · @U12schutz
1171 followers · 35 posts · Server medibubble.orgDie Tagesordnung für das #chmp Meeting der EMA_News ist veröffentlicht. Neben neuen Medikamenten geht es auch wieder um Impfstoffe.
Quelle: https://t.co/6b6jdn4VC8
Für uns relevant werden:
BioNTech: Erweiterung der Zulassung /Seite 14/15
Der erste Punkt bezieht sich auf den bivalenten Impfstoff für die Altersgruppe 5-12.
Der zweite Punkt ist der Nachtrag zur U5 Zulassung vor einigen Wochen.