AkaSci 🛰️ · @AkaSci
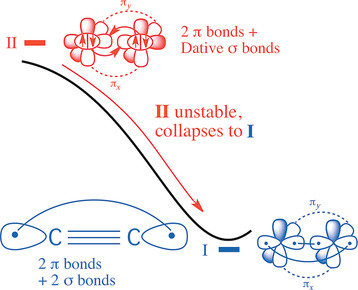
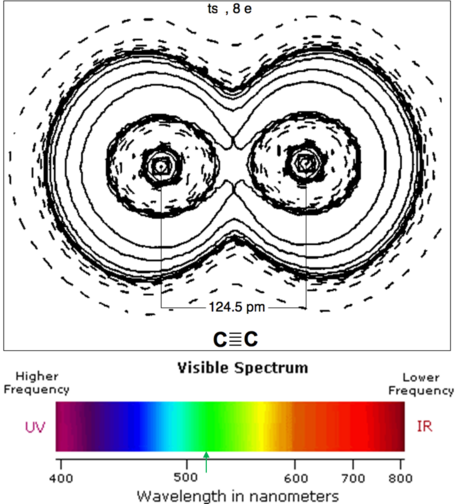
1952 followers · 1907 posts · Server fosstodon.orgSome articles and papers on the enigmatic quadruple bond in C2 -
https://www.chemistryworld.com/news/calculations-reveal-carbon-carbon-quadruple-bond-/3000688.article
https://www.nature.com/articles/nchem.1263
https://chemistry-europe.onlinelibrary
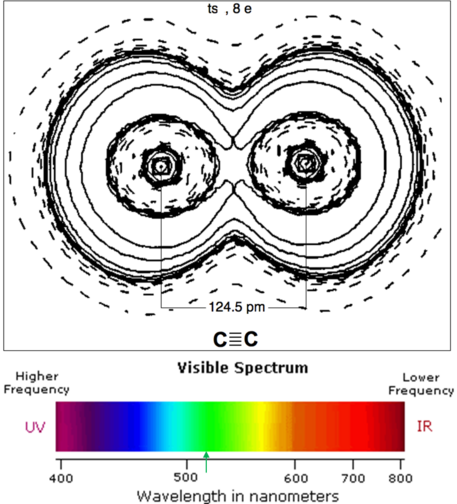
C2 exhibits a triple bond as in N2, but has been shown to have a fourth weaker bond formed by the outer electrons.
AkaSci 🛰️ · @AkaSci
1952 followers · 1906 posts · Server fosstodon.orgDiatomic carbon C2 is a green, gaseous inorganic chemical. It is kinetically unstable at ambient temperature and pressure.
It is found in flames, comets, stars, and the diffuse interstellar medium.
From https://www.pnas.org/doi/10.1073/pnas.2113315118 -
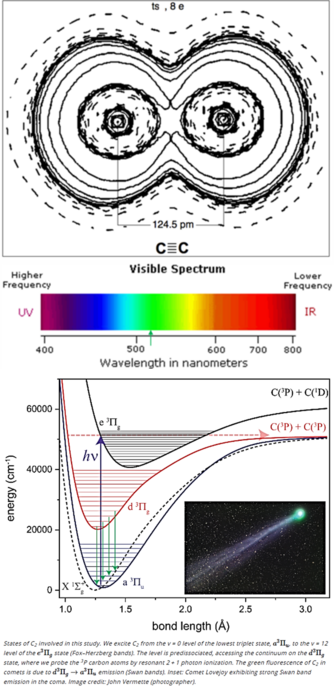
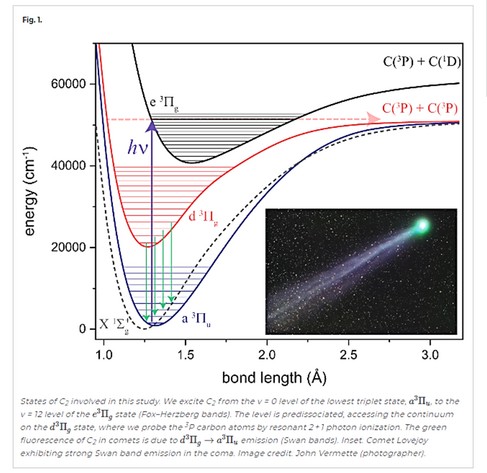
This work shows that, to break the quadruple bond of C2 using sunlight, the molecule must absorb two photons and undergo two “forbidden” transitions.
Oh my!
https://en.wikipedia.org/wiki/Diatomic_carbon
#dicarbon #C2023P1 #Nishimura
9/n
AkaSci 🛰️ · @AkaSci
1946 followers · 1901 posts · Server fosstodon.orgThe green color seen in the coma of most comets, but not in their tails, is due to emissions from quad-bond Diatomic carbon (aka dicarbon) molecules.
Sunlight heats the comet’s ice and organic material to produce C2 molecules, which break apart in ~2 days before they reach the tail. C2 is excited by solar UV radiation and emits mostly in infrared but its triplet state radiates at 518 nm.
https://physicstoday.scitation.org/do/10.1063/pt.6.1.20220110a/full/
https://www.pnas.org/doi/10.1073/pnas.2113315118
#dicarbon #C2023P1 #Nishimura
8/n
AkaSci 🛰️ · @AkaSci
1455 followers · 1561 posts · Server fosstodon.orgThe green color seen in the coma of Comet C/2022 E1 (ATLAS) and other comets, but not in their tails, is due to emissions from Diatomic carbon (aka dicarbon) molecules.
Sunlight heats the comet’s ice and organic material to produce C2 molecules, which break apart in ~2 days before they reach the tail. C2 is excited by solar UV radiation and emits mostly in infrared but its triplet state radiates at 518 nm.
https://physicstoday.scitation.org/do/10.1063/pt.6.1.20220110a/full/
C2 image credit: Omar J. Yepez
#comet #C2023E1 #dicarbon
5/n
AkaSci · @AkaSci
920 followers · 696 posts · Server fosstodon.org@markmccaughrean
The green color seen in the coma of comets incl. that of Comet C/2022 E3 (ZTF) is also related to the elusive quadruple bond of the C2 molecule.
From https://www.pnas.org/doi/10.1073/pnas.2113315118 — "This work shows that, to break the quadruple bond of C2 using sunlight, the molecule must absorb two photons and undergo two 'forbidden' transitions."
Oh my!
Image source: https://www.pnas.org/doi/10.1073/pnas.2113315118
Also see https://fosstodon.org/@AkaSci/109768059428824754
#comet #dicarbon #chemistry
AkaSci · @AkaSci
897 followers · 657 posts · Server fosstodon.orgThe green color seen in the coma of Comet C/2022 E3 (ZTF) is also related to the elusive quadruple bond of the C2 molecule.
From https://www.pnas.org/doi/10.1073/pnas.2113315118 — "This work shows that, to break the quadruple bond of C2 using sunlight, the molecule must absorb two photons and undergo two “forbidden” transitions."
#comet #dicarbon #chemistry
9/n
AkaSci · @AkaSci
897 followers · 656 posts · Server fosstodon.orgThe green color seen in the coma of Comet C/2022 E3 (ZTF) and other comets, but not in their tails, is due to emissions from Diatomic carbon molecules.
Sunlight heats the comet’s ice and organic material to produce C2 molecules, which break apart in ~2 days before they reach the tail. C2 is excited by solar UV radiation and emits mostly in infrared but its triplet state radiates at 518 nm.
https://physicstoday.scitation.org/do/10.1063/pt.6.1.20220110a/full/
#comet #dicarbon #chemistry
8/n
AkaSci · @AkaSci
887 followers · 621 posts · Server fosstodon.org@jhayden
Some articles and papers on the quadruple bond in C2 -
https://www.chemistryworld.com/news/calculations-reveal-carbon-carbon-quadruple-bond-/3000688.article
https://www.nature.com/articles/nchem.1263
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.201600011
From https://www.pnas.org/doi/10.1073/pnas.2113315118
- "This work shows that, to break the quadruple bond of C2 using sunlight, the molecule must absorb two photons and undergo two “forbidden” transitions."
#comet #dicarbon #chemistry
AkaSci · @AkaSci
876 followers · 595 posts · Server fosstodon.orgThe green color seen in the coma of Comet C/2022 E3 (ZTF) and other comets, but not in their tails, is due to emissions from Diatomic carbon (aka dicarbon) molecules.
Sunlight heats the comet’s ice and organic material to produce C2 molecules, which break apart in ~2 days before they reach the tail. C2 is excited by solar UV radiation and emits mostly in infrared but its triplet state radiates at 518 nm.
https://physicstoday.scitation.org/do/10.1063/pt.6.1.20220110a/full/
C2 image credit: Omar J. Yepez
#comet #dicarbon #chemistry
4/n