rfhb · @rfhb
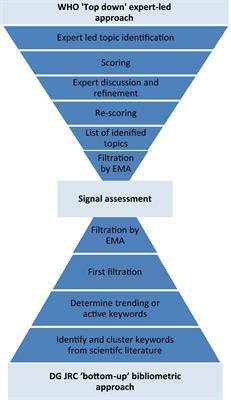
1 followers · 5 posts · Server mastodon.onlineNew from our #HorizonScanning of #RegulatoryScience #Health #Innovation at the European Medicines Agency: snapshots of challenges & opportunities from medicines regulatory perspective for 25 technologies identified working with the World Health Organization & European Commission JRC: https://doi.org/10.3389/fmed.2022.1064003
#innovation #health #regulatoryscience #HorizonScanning
rfhb · @rfhb
1 followers · 5 posts · Server mastodon.onlineTech note: for clinical trial information from registers, R package ctrdata has been updated to also work with https://duckdb.org/ and to further support retrieval and analysis https://cran.r-project.org/package=ctrdata v1.11.1 #ClinicalTrials #ClinicalResearch #RegulatoryScience #MethodsResearch #MeidcinesInnovation
#meidcinesinnovation #methodsresearch #regulatoryscience #clinicalresearch #clinicaltrials
rfhb · @rfhb
1 followers · 5 posts · Server mastodon.onlineFirst toot: to flag an interesting scoping review on non-concurrent controls in platform clinical trials https://arxiv.org/abs/2211.09547 by IMI EU-PEARL https://twitter.com/martabofillr https://twitter.com/posch_m https://twitter.com/KH_Stats #IMI_EU-PEARL suggesting some methods are available, guidance is limited #RegulatoryScience #ControlledTrials #PlatformTrials #MedicineDevelopment #MethodsResearch #ClinicalResearch
#clinicalresearch #methodsresearch #medicinedevelopment #platformtrials #controlledtrials #regulatoryscience #imi_eu